

PROCRIT® is indicated for the treatment of anemia in patients with non-myeloid malignancies where anemia is due to the effect of concomitant myelosuppressive chemotherapy, and upon initiation, there is a minimum of two additional months of planned chemotherapy.
PROCRIT® has not been shown to improve quality of life, fatigue, or patient well-being.
PROCRIT® is not indicated for use:
In anemic patients with cancer with non-myeloid malignancies receiving myelosuppressive chemotherapy
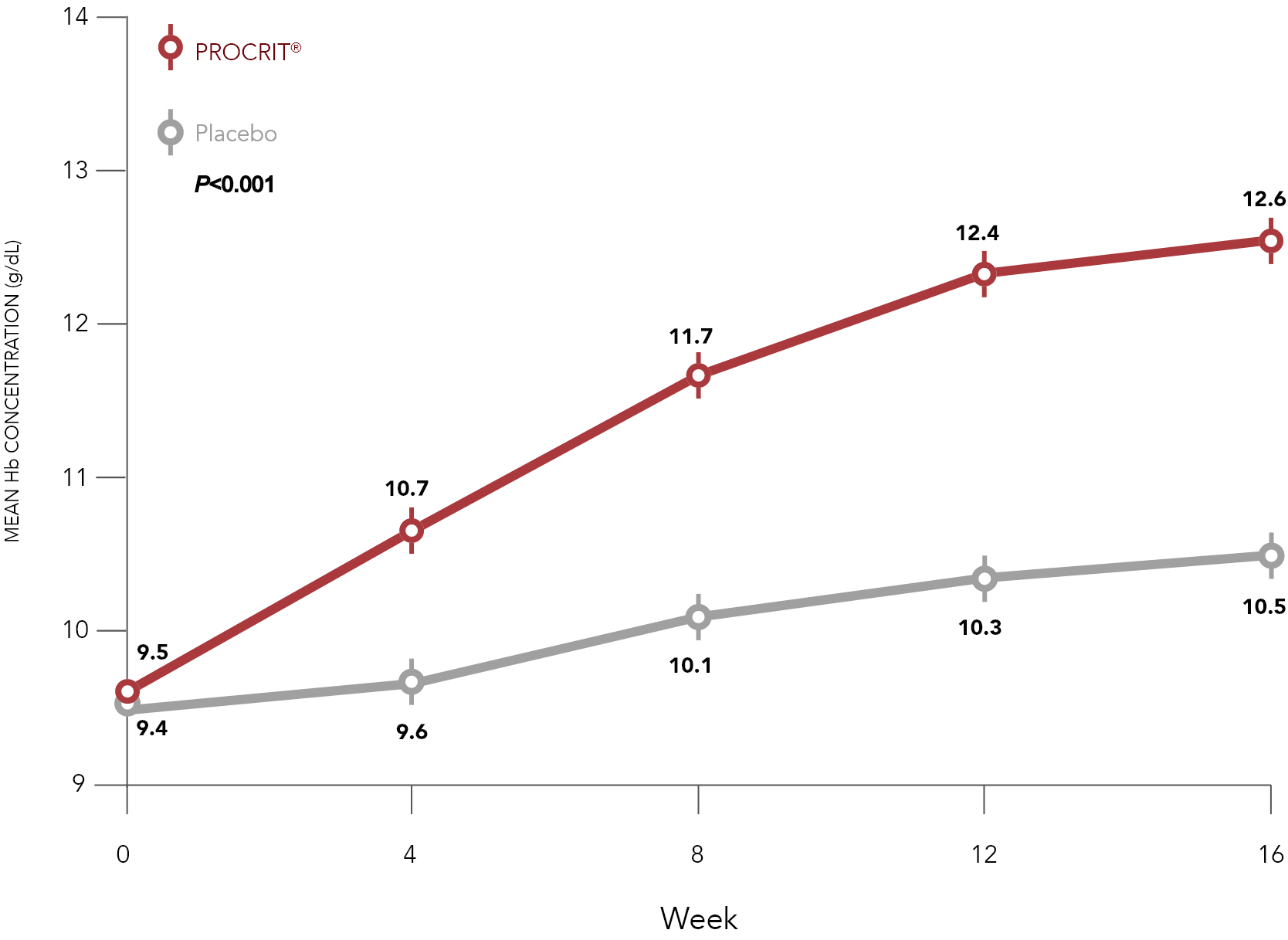
Change in mean Hb values by week on study2
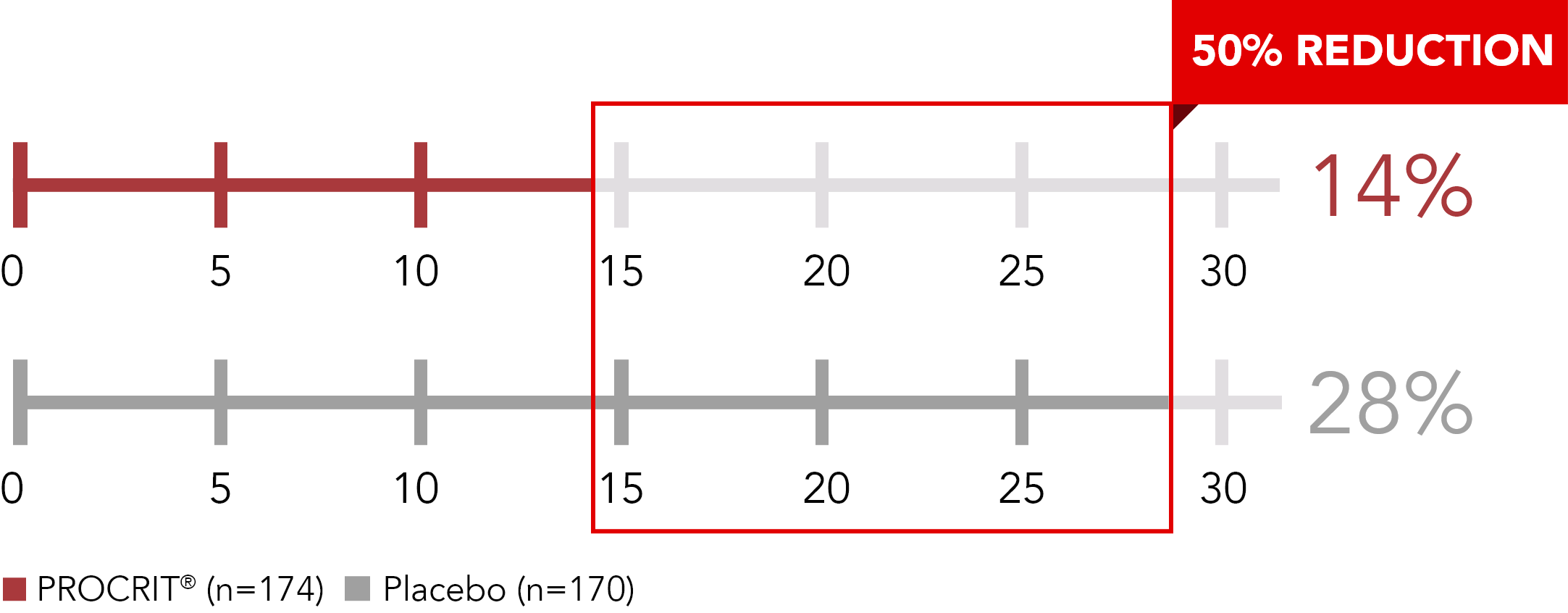
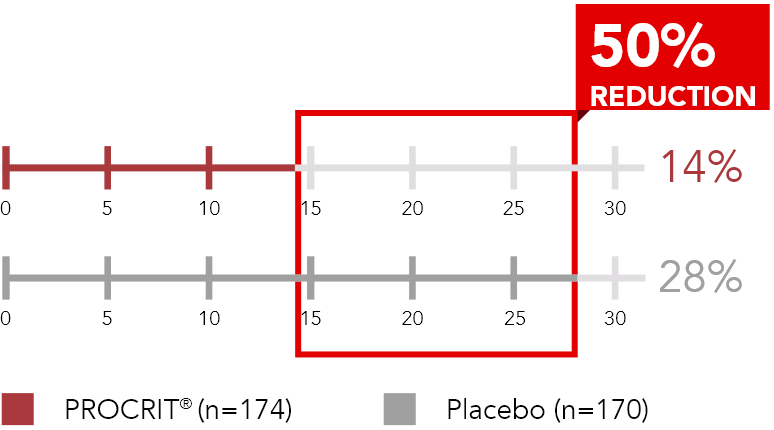
Proportion of patients transfused, Week 5 through Week 16 or end of study
| PATIENTS | DESIGN | TREATMENT |
|---|---|---|
|
|
|
| PATIENTS |
|
|---|---|
| DESIGN |
|
| TREATMENT |
|
WARNINGS: ESAs INCREASE THE RISK OF DEATH, MYOCARDIAL INFARCTION, STROKE, VENOUS THROMBOEMBOLISM, THROMBOSIS OF VASCULAR ACCESS AND TUMOR PROGRESSION OR RECURRENCE
Cancer:
Expand the Important Safety Information at the bottom of the page to see the complete Boxed Warnings.
The adverse reactions with a reported incidence of ≥5% in PROCRIT®-treated patients that occurred at a higher frequency than in placebo-treated patients are shown in the table below:
Adverse reactions in patients with cancer1
| ADVERSE REACTION | PROCRIT®-treated patients (n=168) | Placebo-treated patients (n=165) |
|---|---|---|
| NAUSEA | 35% | 30% |
| VOMITING | 20% | 16% |
| MYALGIA | 10% | 5% |
| ARTHRALGIA | 10% | 6% |
| STOMATITIS | 10% | 8% |
| COUGH | 9% | 7% |
| WEIGHT DECREASE | 9% | 5% |
| LEUKOPENIA | 8% | 7% |
| BONE PAIN | 7% | 4% |
| RASH | 7% | 5% |
| HYPERGLYCEMIA | 6% | 4% |
| INSOMNIA | 6% | 2% |
| HEADACHE | 5% | 4% |
| DEPRESSION | 5% | 4% |
| DYSPHAGIA | 5% | 2% |
| HYPOKALEMIA | 5% | 3% |
| THROMBOSIS | 5% | 3% |